There are two main types of clinical studies. Resolving ethical issues in stem cell clinical trials.
Ethical Issues In The Development Of Readiness Cohorts In Alzheimer S Disease Research The Journal Of Prevention Of Alzheimer S Disease

Ethical Issues In Clinical Research Topic Of Research Paper In Clinical Medicine Download Scholarly Article Pdf And Read For Free On Cyberleninka Open Science Hub

Clinical Trials Ethical Issues And Patient Recruitment Bmj Group
The Phase 1 Advisory Group discusses topics including operational issues for the review of Phase 1 trials training for REC members in ethical review of Phase 1 trials and initiatives to improve the efficiency and effectiveness of ethical review.
Ethical issues in clinical trials. This Clinical Trials Conference includes a wide range of Keynote presentations Plenary talks Symposia Workshops Exhibitions Poster presentations and Career development programs. Clinical trials conducted in Australia are subject to various regulatory controls to ensure the safety of participants. Transition from the Clinical Trials Directive to Regulation.
Clinical trials also called interventional studies and observational studies. J Law Med Ethics. The example of Parkinson disease.
Such tenets may allow doctors care providers and families. The practice of tele-mental health. Additional ethical requirements are also warranted to strengthen trial design coordinate scientific and ethics review verify that participants understand key features of the trial and ensure publication of negative.
Medical ethics is an applied branch of ethics which analyzes the practice of clinical medicine and related scientific research. This scoping review aims to identify and integrate evidence on the ethical issues reported when recruiting retaining and tracing families and. RCTs are ethical only in conditions of clinical equipoise being assured.
And Related Issues in Clinical Trials This guidance is intended to assist applicants in choosing a control group for clinical trials. Special protections for children as research subjects. We regulate the use of therapeutic goods supplied in clinical trials in Australia under the therapeutic goods legislation.
213 Ethical issues. Conference series LLC Ltd Organizes 3000 Global Events Every Year. Barnett JE Kolmes K.
These clinical trials should follow ethical principles that guide all clinical research including appropriate balance of risks and benefits and informed voluntary consent. Clinical trial sponsors must be aware of the requirements to import export manufacture and supply therapeutic goods in Australia. ClinicalTrialsgov includes both interventional and observational studies.
In any hSC research however difficult dilemmas arise regarding sensitive downstream research consent to donate materials for hSC research early clinical trials of hSC therapies and oversight of hSC research. Clinical Trials 2022. CrossRef Full Text.
14 were in human trials. ACRP-CP ACRP Certified Professional is a new credential formally recognizing professionals involved in all aspects of clinical studiestrials who have demonstrated the knowledge skills and abilities to perform ethical and responsible clinical research by passing the standardized ACRP Certified Professional Certification Exam. Choice of Control Group in Clinical Trials Step 5 NOTE FOR GUIDANCE ON CHOICE OF CONTROL GROUP IN CLINICAL TRIALS CPMPICH36496 TRANSMISSION TO CPMP June 1999 RELEASE FOR CONSULTATION June 1999 DEADLINE FOR COMMENTS December 1999 FINAL.
The environment in which clinical trials are conducted is complex often occurring across multiple jurisdictions and with every study needing ethics and governance approvals before it can commence. A clinical study involves research using human volunteers also called participants that is intended to add to medical knowledge. Under the Declaration of Helsinki patients in clinical trials must participate entirely voluntarily and must have the right to leave the trial at any timeThis ethical imperative makes missing data an inevitable problem of clinical trials and requires appropriate analysis methods to account for it.
Lo B Parham L. Ethical issues in tuberculosis vaccine trials. Ethical legal and clinical issues for practitioners.
This Directive will be repealed on the day of entry into application of the Clinical Trials Regulation. Fetal cell lines have been widely used in basic science and clinical medicine for decades. Every clinical trial has a protocol or action plan for conducting the trial.
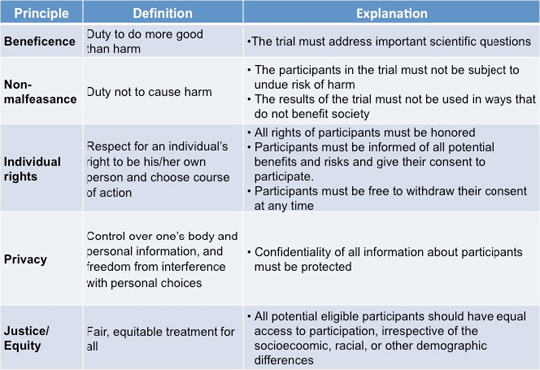
These values include the respect for autonomy non-maleficence beneficence and justice. Clinical equipoise and randomized clinical trials RCTs RCT is a study design that randomizes whether the participants are given treatment or placebo for the sake of eliminating prejudice. Clinical trials are an essential part of the process of evidenced based practice and can help guide treatment decisions for both health care professionals and patients.
Clinical trials benefit patients advance medical knowledge and are estimated to be worth around 1 billion to the Australian economy each year. How making a COVID-19 vaccine confronts thorny ethical issues. Use of financial incentives in two low-risk randomized clinical trials did not present ethical issues and in one of the trials the incentives increased study participation according to findings.
Department of Health and Human Services. The reprogramming of somatic cells to produce induced pluripotent stem cells avoids the ethical problems specific to embryonic stem cell research. All reproductive medicine units offering ART services should comply with the Ethical guidelines on the use of assisted reproductive technology in clinical practice and research.
Until the Clinical Trials Regulation EU No 5362014 will become applicable all clinical trials performed in the European Union are required to be conducted in accordance with the Clinical Trials Directive. Pract Innovations 2016 115366. 29 This guidance discusses the scientific and ethical issues that should be addressed when 30 considering the inclusion of pregnant women in drug development clinical trials.
Ethical Issues 213. Clinical trials may also compare a new treatment to a treatment that is already available. Assisted Reproductive Technology ART Assisted reproductive technology ART is the application of laboratory or clinical technology to gametes human egg or sperm andor embryos for the purposes of reproduction.
Article PubMed Google Scholar 2. TBC Minutes of previous meetings Minutes. Clinical trials are an important part of the pathway by which new medicinal products can obtain a licence from MHRA and become available for use as a new treatment in patients.
Background The internet is an increasingly popular tool in family and child research that is argued to pose new ethical challenges yet few studies have systematically assessed the ethical issues of engaging parents and children in research online. One of the most important problems in analyzing a clinical trial is the occurrence of the dropout. Clinical trials are conducted to collect the data necessary to provide information for academia industry and regulators to make decisions about the safety and efficacy of the disease illness or preventative medicines under study.
Medical ethics is based on a set of values that professionals can refer to in the case of any confusion or conflict. The plan describes what will be done in the study how it will be conducted and why each part of the study is necessary. CLINICAL CASE STUDY SERIES Quality Management in Clinical Trials.
10 th International Conference on Clinical Trials is scheduled to be held during August 04-05 2022 at Vancouver Canada.

Clinical Trial Ethics Protocol Informed Consent And Study Conduct

Ethical Issues In Clinical Trials In Children Download Table
Cisn How Cancer Is Studied Ethical Issues
Clinical Trials Two Neglected Ethical Issues Abstract Europe Pmc

Spirit 2013 New Guidance For Content Of Clinical Trial Protocols The Lancet

Ethics In Clinical Trials

Ethical Considerations In Clinical Trials
Oatext Com