In a fatty acid chain if there are only single bonds between neighboring carbons in the hydrocarbon chain the fatty acid is said to be saturated. You will learn more about these different types of hydrocarbons later in this chapter.
Saturated Vs Unsaturated Hydrocarbons Definition 10 Key Differences Examples
Difference Between Saturated And Unsaturated Hydrocarbons Definition Structure Types Properties
Saturated And Unsaturated Hydrocarbons Contemporary Organic Synthesis Rsc Publishing
CCEA Double award science.

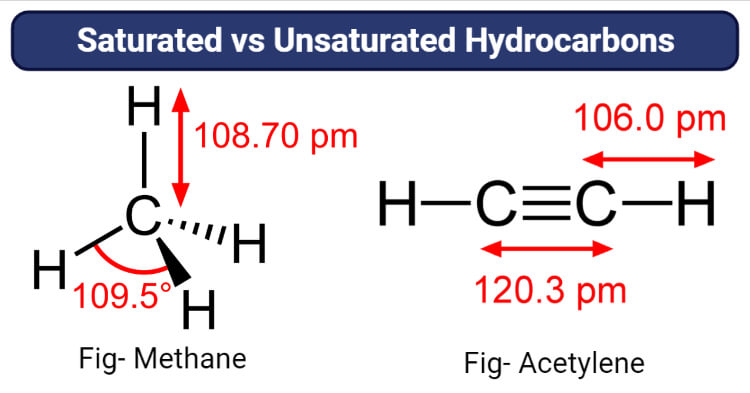
Saturated and unsaturated hydrocarbons. Saturated Hydrocarbon A hydrocarbon is said to be saturated if it contains only CC single bonds. Unsaturated hydrocarbons are more likely to be solid than their saturated counterparts as are cyclic hydrocarbons. It is impossible to add more hydrogen atoms to the compound so it is saturated with hydrogen.
A hydrocarbon that has at least one double or triple bond between carbon atoms is an unsaturated hydrocarbon. Describe the reactions characteristic of saturated and unsaturated hydrocarbons Identify structural and geometric isomers of hydrocarbons The largest database 1 of organic compounds lists about 10 million substances which include compounds originating from living. Some hydrocarbons prevent the rotation of the atoms about the bond by locking them into specific structural formations.
Interconversion of Saturated and Unsaturated Solution. The Saturated Hydrocarbons or Alkanes. Alkanes are saturated their carbon atoms are only joined by C-C single bonds alkenes are unsaturated they contain at least.
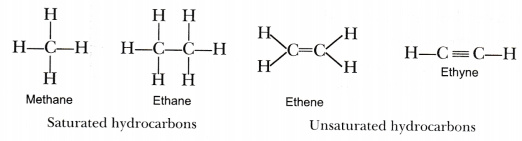
Saturated hydrocarbons contain carbon-carbon and carbon-hydrogen single bonds. Reading Check Explain the origin of the terms saturated and unsaturated hydrocarbons. Alkanes hydrocarbons in which all the bonds are single have molecular formulas that satisfy the general.
Fatty acids may be saturated or unsaturated. Unsaturated hydrocarbons are hydrocarbons that have double or triple covalent bonds between adjacent carbon atomsThe term unsaturated means more hydrogen atoms may be added to the hydrocarbon to make it saturated ie. An organic compound consisting entirely of hydrogen and carbon.
Hydrocarbons in Fuel Hydrocarbons containing between six and 10 carbon molecules are the top components of most fuels regardless of whether they are alkanes alkenes or cyclic. Most of the time the bond exists in the form of a covalent bond. Compounds that contain only carbon and hydrogen are known as hydrocarbonsThose that contain as many hydrogen atoms as possible are said to be saturatedThe saturated hydrocarbons are also known as alkanes.
Alkanes and alkenes both form homologous series of hydrocarbons but. Alkanes are described as saturated hydrocarbons while alkenes alkynes and aromatic hydrocarbons are said to be unsaturated. On the other hand if carbon atoms.
Contain fewer hydrogens than the corresponding saturated hydrocarbon. As a result of this more solute can be dissolved into the solvent. Aliphatic compounds may be saturated or unsaturated.
Further Chemical Reactions Rates and Equilibrium Calculations and Organic. Aromatic compounds originally named because of their fragrant properties are unsaturated hydrocarbon ring structures that exhibit special properties including unusual stability. Experiment 3 Hydrocarbons Page 2 Hydrocarbons may be saturated or unsaturatedA saturated hydrocarbon is one that is maxed out in terms of the number of hydrogens that can be present given the number of carbons in the compound.
Ii unsaturated and iii aromatic hydrocarbons. Consisting all single bonds. Hydrocarbons Class 11 Notes Chemistry Chapter 13 Hydrocarbon A compound of carbon and hydrogen is known as hydrocarbon.
On heating the saturated solution the solubility of that particular solute increases in the given solvent. There are no double or triple bonds in the molecules. Examples include alkanes and cycloalkanes.
The simplest alkane is methane. Hydrogenation can be used to create a fully saturated ring system. 258 explain that cracking involves the breakdown of larger saturated hydrocarbons alkanes into smaller more useful ones some of which are unsaturated alkenes.
Vinegar is an unsaturated acetic acid solution in water. Having only single bonds is defined as a saturated hydrocarbon. Saturated Hydrocarbons Alkanes and Cycloalkanes.
Contain more hydrogen atoms than the corresponding unsaturated hydrocarbons. Most reactions of organic compounds take place at or adjacent to a functional groupIn order to establish a baseline of behavior against which these reactions may be ranked we need to investigate the reactivity of. Saturated fatty acids are saturated with hydrogen since single bonds increase the number of hydrogens on each carbon.
The higher melting points of the saturated fatty acids reflect the uniform rod-like shape of their molecules. An acyclic saturated hydrocarbon with the general formula C n H 2n2Also called paraffin. Saturated and Unsaturated Aliphatic Hydrocarbons.
The configuration of an unsaturated carbons include straight chain such as alkenes and alkynes as well as branched chains and aromatic compounds. Examples of Unsaturated Solutions. This means alkenes and alkynes more likely to readily react with a chemical reagent than an alkane.
They contain sp 2 or sp hybridized carbons. For hydrocarbons the DBE or IHD tells us the number of rings andor extra bonds in a non-saturated structure which equals to the number of hydrogen pairs that are required to make the structure saturated simply because joining two elements to form a ring or adding one extra bond in a structure reduces the need for two Hs. Hydrocarbons with only carbon-to-carbon single bonds CC and existing as a continuous chain of carbon atoms also bonded to hydrogen atoms are called alkanes or saturated hydrocarbons.
Ethane CH3CH3 Unsaturated Hydrocarbon Aromatic Hydrocarbon Benzene and its derivatives are called aromatic compounds. Definitions of organic compounds. An unsaturated hydrocarbon that contains at least one carboncarbon double bond with the general formula C n H 2n.
Saturated hydrocarbons can be distinguished from unsaturated hydrocarbons in the laboratory because saturated hydrocarbons are less chemically active reactive than unsaturated hydrocarbons. 2516 determine the presence of a CC using bromine water. Saturated solution on heating becomes unsaturated whereas an unsaturated solution becomes saturated upon cooling.
If different carbon atoms are joined together to form open chain of carbon atoms with single bonds they are termed as alkanes as you have already studied in Unit 12. A unsaturated Even though rings only contain single bonds rings are considered unsaturated b unsaturated c saturated d unsaturated 2. Adding a spoonful of sugar to a hot cup of coffee produces an unsaturated sugar solution.
Main groups of hydrocarbons. The cis-double bonds in the unsaturated fatty acids introduce a kink in their shape which makes it more difficult to pack their molecules together in a stable repeating array or crystalline lattice. The main difference between saturated and unsaturated hydrocarbon is that saturated hydrocarbons contain only single covalent bonds between carbon atoms whereas unsaturated hydrocarbons contain at least one double or triple covalent bond in the main chain.
Saturated in this case means that each carbon atom is bonded to four other atoms hydrogen or carbonthe most possible. If the molecular structure is given the easiest way to solve is to count the number of double bonds triple bonds andor rings. CH 4The Lewis structure of methane can be generated by combining the four electrons in the valence shell of a.
Saturated hydrocarbon contains mainly of alkanes which are open chain hydrocarbons containing carbon-carbon single bond. All carbon atoms are sp 3 hybridized in these compounds.
What Are Hydrocarbons Give Examples Cbse Class 10 Science Learn Cbse Forum

Difference Between Saturated And Unsaturated Hydrocarbon Javatpoint

Saturated Vs Unsaturated Hydrocarbons Labster Theory

Petroleum Refining Saturated Molecules Britannica

Ppt Unsaturated Hydrocarbons Powerpoint Presentation Free Download Id 6511501

Solved Unsaturated Hydrocarbons Section 13 1 13 1 Classify Chegg Com
Saturated And Unsaturated Hydrocarbons Contemporary Organic Synthesis Rsc Publishing

Unsaturated Hydrocarbon Bartleby


